the tendency of an atom to attract electrons|Electronegativity (video) : Tuguegarao Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the . Language translation with voice, text, camera image translator. Use offline
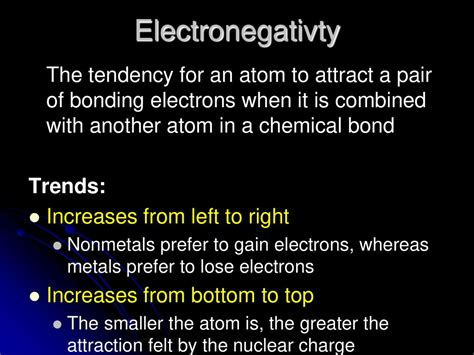
the tendency of an atom to attract electrons,Definition. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is .Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. It determines how the shared electrons are distributed . Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the .the tendency of an atom to attract electrons Electronegativity is a chemical property that describes the tendency of an atom or a functional group to attract electrons toward itself. The electronegativity of an atom .Electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituen. Electronegativity is a measure of an atom's ability to attract shared electrons to itself. On the periodic table, electronegativity generally increases as you .the tendency of an atom to attract electrons Electronegativity (video) Electronegativity is a measure of an atom's ability to attract shared electrons to itself. On the periodic table, electronegativity generally increases as you .Electronegativity is a property that describes the tendency of an atom to attract electrons (or electron density) toward itself. An atom’s electronegativity is affected by .Chem 1201. Unit 2: Periodic Properties of the Elements. 2.12: Electronegativity. Expand/collapse global location. 2.12: Electronegativity. Page ID. Learning Objectives. .Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. It determines how the shared electrons are distributed .The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is known as electronegativity. It is a dimensionless property because it is only a tendency. It basically indicates the net .What is the tendency of an atom to attract a pair of electrons that will bond? Polar covalent. It increases. . 2 of 3. Term. Where there is unequal sharing between the two atoms of electrons in a bond, the bond is _____. Polar covalent. Electronegativity. It increases. 3 of 3. Term. What is the tendency of an atom to attract a pair of .Electronegativity Definition. Electronegativity is a chemical property that measures the tendency of an atom to attract electrons towards itself. Electronegativity is affected by the atomic number and the distance .
The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is known as electronegativity. It is a dimensionless property because it is only a tendency. It basically .

Figure 6.5.1 6.5. 1: Periodic Table of Electronegativity values. Electronegativity measures an atom's tendency to attract and form bonds with electrons. This property exists due to the electronic configuration of atoms. Most atoms follow the octet rule (having the valence, or outer, shell comprise of 8 electrons).
Electronegativity (video) Definition. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7.
Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) towards itself. It determines how the shared electrons are distributed between the two atoms in a bond. The more strongly an atom attracts the electrons in its bonds, the larger its electronegativity. .
Electronegativity. Electronegativity is a property that describes the tendency of an atom to attract electrons (or electron density) toward itself. An atom’s electronegativity is affected by both its atomic number and the size of the atom. The higher its electronegativity, the more an element attracts electrons.

(iv) The tendency of an atom to attract electrons to itself when combined in a compound. (v) The reaction in which carboxylic acid reacts with alcohol in the presence of conc. H 2 SO 4 to form a substance having a fruity smell. Study Material. Chemistry. Give one word or a phrase for the following statements: .Electronegativity. Molecules are collections of atoms that are associated with one another through bonds. It is reasonable to expect — and the case empirically — that different atoms will exhibit different physical properties, including abilities to interact with other atoms. One such property, the tendency of an atom to attract electrons, is described by the . This is a discussion on what electronegativity, or the tendency of an atom to attract electrons, does to bonding chemistry. Those with even electronegativities tend to form more covalent bonds, whereas those with more stark differences, form ionic bonds. Metallic bonds form when all relevant elements desire to let go of their electrons, which .Which of the following atoms has the greatest tendency to attract electrons? 1. barium 2. beryllium 3. boron 4. bromine; Generally, elements with very negative electron affinities gain electrons easily to form negative ions. Which group of .
Because the tendency of an element to gain or lose electrons is so important in determining its chemistry, various methods have been developed to quantitatively describe this tendency. . The electronegativity (χ) of an element is the relative ability of an atom to attract electrons to itself in a chemical compound and increases diagonally .Electronegativity is a chemical property that describes the tendency of an atom or a functional group to attract electrons toward itself. The electronegativity of an atom is affected by both its atomic number and the distance that its valence electrons reside from the charged nuclei. The concept of electronegativity was first proposed by Pauli in .Electronegativity is defined as the ability of an atom in a particular molecule to attract electrons to itself. The greater the value, the greater the attractiveness for electrons. Electronegativity is a function of: the atom's ionization energy (how strongly the atom holds on to its own electrons) and. You will discover that knowing how to use the periodic table is the single most important skill you can acquire to understand the incredible chemical diversity of the elements. 8.1: Electromagnetic Radiation. 8.2: Atomic Spectra. The photoelectric effect provided indisputable evidence for the existence of the photon and thus the particle-like .Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus.Electronegativity is a measure of the tendency of an atom to attract electrons (or electron density) within a bond. Electronegativity differences can be used to predict how shared electrons are distributed between the two nuclei in a bond. The more strongly an atom attracts the electrons within its bonds, the larger its electronegativity value.
the tendency of an atom to attract electrons|Electronegativity (video)
PH0 · electronegativity
PH1 · Electronegativity – Introductory Chemistry
PH2 · Electronegativity (video)
PH3 · Electronegativity
PH4 · 7.2 Covalent Bonding
PH5 · 2.12: Electronegativity
PH6 · 10.3: Covalent bonding and Electronegativity